
 |
| Home | About | Software | Curriculum | Research |
| Contents of About: About the Project | Proposal |
Home Page ![]() About CC-ATOMS
About CC-ATOMS ![]() Proposal
Proposal ![]() Learning from Models
Learning from Models
| Proposal Contents: |
INTRODUCTION
To understand much of modern technology, students need robust
mental models of atoms, molecules, and their interactions. Many
of the areas of greatest job growth, such as biotechnology, medical
technologies, nursing and allied health occupations such as nutrition,
microcircuits, and photonics, depend on exploiting the properties
of atoms and molecules. Almost half of the fastest growing jobs
(13 out of 30), for example, are in biological sciences and health
related fields (CT Dept. of Labor, 2001). To prepare students
to understand future technologies in areas such as these, they
need to be conversant with atomic-scale interactions and how these
determine macroscopic phenomena. This is particularly true as
the chemical basis of living systems becomes better understood
and more important in areas as diverse as law, forensics, farming,
and nano-engineering.
Learning from Models
There is considerable evidence that students have major misconceptions
about atoms and molecules and few ideas about how the forces and
motions at this scale relate to macroscopic properties. Even though
they are familiar with the idea of atoms, many students believe
that there is some continuous matter between atoms and that the
properties of atoms such as color, strength, and stickiness, are
simply identical to macroscopic properties (Anderson, 1990; Lee,
et al 1993; Nussbaum 1985). For instance, students believe that
the molecules making up paper would catch fire and that molecules
of ice are cold. Furthermore, few students are aware of electrostatic
forces and their dominant role at the atomic scale (Anderson,
1990; Children's Learning in Science, 1987).
Initial studies have demonstrated the educational value of highly
interactive research-based models of atomic and molecular systems.
(Hakerem, 1994; 1996; Birk, 1997; Tinker, 2001a) Surprisingly
complex models of atoms and molecules can now be run on the inexpensive
desktop computers that are widely available to students. From
a few basic potentials and rules, these models can compute the
motion of a few hundred atoms while simultaneously displaying
a representation of the motion of the ensemble. This can be done
quickly enough for students to see the motion and explore the
effects of various parameters on the evolution of many systems.
Using such models, we can demonstrate the phases of matter, the
gas laws, phase change, latent heat, diffusion, gas absorption,
osmosis, thermal diffusion, conformational changes, and the properties
of liquid crystals. Student explorations of these models lead
to a good understanding of connections between atomic-scale events
and what they observe at the macroscopic scale. Using these models
and supporting curricula, students are able to predict new micro-macro
connections that they have not previously encountered (Tinker,
2001c).
These dynamic, interactive models create very powerful learning
experiences. It is hard for instance, to understand osmosis from
a written or verbal description of diffusion through a semi-permeable
membrane, but it is almost obvious when you see a dynamic model
of it actually happening. The learning experience is particularly
effective because the model is more than an animation and students
can experiment with the system to find out how it "works",
that is, what combinations of molecule size, density, pore size,
temperature contribute to osmotic pressure. This creates the kind
of rich, highly associated mental network of concepts that we
know contributes to lasting understanding (Snir and Smith,1995;
Jackson, et al, 1994).
Models of Atomic-Scale Interactions
Our work to date has concentrated on models that explain phenomena
encountered primarily in physics and physical chemistry. This
has resulted in the Molecular Workbench model currently
being tested with students. To exploit the educational value of
our modeling-based approach in areas that depend on chemistry,
biology, and biochemistry, we need to extend the Molecular
Workbench model to include covalent bonds, photon interactions,
conformation, and other functions. These will permit students
to explore a range of new phenomena such as chemical reactions,
equilibria, reaction rates, catalysis, molecular form and function,
color, spectra, phosphorescence, and fluorescence, many in three
dimensions.
 These additions to Molecular
Workbench will require major technical advances in computational
models. There are no general molecular dynamics models that include
chemical reactions and light-photon interactions. While creating
such models sounds ambitious, the reason that these additions
are feasible in this project is that we are not building a research
model; we can judiciously trade off precision for educational
value. For instance, we do not need to model a quantum mechanical
solution of covalent bonds. We have already created a heuristic
model of monovalent reactions that conserves energy. This is sufficiently
rich to model, for instance, the role of energy and entropy differences
in shifting chemical equilibria, a set of important ideas that
most students currently must take on faith. We plan to incorporate
similar heuristic rules for multi-valent bonding and photon interactions
that support the development of accurate student concepts while
avoiding some of the confusing complexity of more complete solutions.
These additions to Molecular
Workbench will require major technical advances in computational
models. There are no general molecular dynamics models that include
chemical reactions and light-photon interactions. While creating
such models sounds ambitious, the reason that these additions
are feasible in this project is that we are not building a research
model; we can judiciously trade off precision for educational
value. For instance, we do not need to model a quantum mechanical
solution of covalent bonds. We have already created a heuristic
model of monovalent reactions that conserves energy. This is sufficiently
rich to model, for instance, the role of energy and entropy differences
in shifting chemical equilibria, a set of important ideas that
most students currently must take on faith. We plan to incorporate
similar heuristic rules for multi-valent bonding and photon interactions
that support the development of accurate student concepts while
avoiding some of the confusing complexity of more complete solutions.
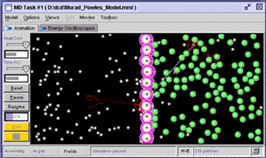
Figure 1. A Molecular Workbench model of osmosis. The vertical row of atoms in the center that make up the membrane are tied to the background with harmonic constraints.
Pedagogical Support
A sophisticated model is not, by itself, much use in education.
If students are asked to learn by exploring such models, they
generally become lost and bored. If they bravely persist, they
may discover some of the underlying science, but will likely miss
critical parts and fail to transfer their knowledge to other situations
or to tests. The model needs to be incorporated into instructional
activities that employ effective learning strategies (Bent, 1984;
Buckley, 2000; Harrison & Treagust, 1996; Horwitz, 1995-6;
Horwitz & Christie 1999; Lee, et al, 1993: Snir and Smith,
1995).
Computer-based learning environments can make inquiry-based learning
more successful by offering students cognitive and procedural
guidance and by freeing teachers to interact with students about
complex science topics (diSessa, 1992; Vanderbilt, 1997; Feurzeig
& Roberts, 1999; Slotta & Linn, 1999; Linn & Hsi,
2000; White & Frederickson, 1998). This is important because
the best learning strategy for use with complex models is to provide
opportunities for student learning through guided inquiry in an
engaging social context (Glaser, 1976; Champagne, et al, 1980;
Vanderbilt, 1997; Driver, 1985; Collins, et al, 1991; Scardamalia
& Bereiter, 1992; Brown & Campione, 1994; White &
Frederickson, 1998). The guidance typically involves giving students
a question or challenge, providing a simplified version of the
model to explore, asking for predictions, probing for reflection,
and providing help if the student is confused. Guided exploration
of this kind converts a model into a learning activity.
The proposing partners have developed two complementary technologiesPedagogica
and WISEthat support inquiry-based guided explorations of
models. Pedagogica is an application that allows curriculum
developers to write scripts that can make many specific scaffolded
models called "hypermodels" (Horwitz & Tinker, 2001;
Tinker, 2001d). Many scaffolded hypermodels can be derived from
one complex model such as the Molecular Workbench. Individual
hypermodels provide help in exploring and understanding a specific
aspect of a model.
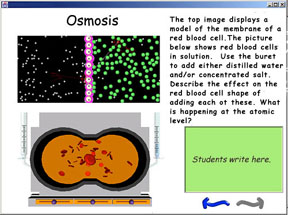 For example, as mentioned
above, one of the many emergent phenomena that Molecular Workbench
software can model is osmosis. Figure 1 shows Molecular Workbench
software configured for explorations of osmosis. However, as part
of a lesson, students may need to focus on just one aspect of
the system, such as the relative sizes of the molecules. Pedagogica
can be used to create a hypermodel for such a lesson with only
the needed controls, supporting text, and a coordinated macroscopic
model, as shown in Figure 2.
For example, as mentioned
above, one of the many emergent phenomena that Molecular Workbench
software can model is osmosis. Figure 1 shows Molecular Workbench
software configured for explorations of osmosis. However, as part
of a lesson, students may need to focus on just one aspect of
the system, such as the relative sizes of the molecules. Pedagogica
can be used to create a hypermodel for such a lesson with only
the needed controls, supporting text, and a coordinated macroscopic
model, as shown in Figure 2.
WISE, as illustrated in Figure 3, provides a friendly, teacher-controlled server-side environment for combining hypermodels with other online resources into complete instructional modules that can be delivered entirely on the Web (Slotta & Linn, 2000). Together, Pedagogica and WISE create a powerful learning and distribution system that exploits and delivers the educational value of models such as those developed in the Molecular Workbench project.

This material is based upon work supported by the National Science Foundation under Grant No. EIA-0219345. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Last Update: 12/10/2018 Maintainer: webmaster@concord.org
Site Map
Copyright © 2018, The Concord Consortium.
All rights reserved.